Concerning Questions - Interview with UK Column
Regulatory failure, the revolving door and how dangerous is PROCESS 2?
During our discussion in a recorded interview with UK Column, we covered a range of important topics. UK COLUMN VIDEO
Compressed Phase 1 Study and Safety Concerns
First, we delved into the safety concerns surrounding the Pfizer 'vaccine'. It was revealed that despite the novel LNP/mRNA platform being extensively researched for 30 years, it had been deemed unsafe and not utilized due to its minimal safety testing. This raised serious questions about the safety of the Pfizer vaccine. The manufacture and distribution of LNP (lipid nanoparticles) also raised concerns, as the process involves intricate steps that need to be closely monitored to ensure safety and efficacy.
What did they know and when did they know it?
It is a question that is difficult to find answers to, and now even freedom of information (FOI) requests get turned down for minimal reasons.
The MHRA, EMA, FDA, and CDC - how did they share information?
We talked about regulatory concerns, particularly between organizations such as the MHRA, EMA, FDA, and CDC.
The WarRoom/DailyClout Pfizer Document Investigation volunteers
Another aspect we discussed was the commendable work carried out by the WarRoom/DailyClout Pfizer Document Analysis volunteers. Their commitment to analyzing Pfizer documents helped shed light on various important issues.
Additionally, we touched upon the collaborative efforts of a dedicated team working on scientific manuscripts. Their research and analysis contribute significantly to our understanding of various aspects related to vaccines and their effects.
PROCESS 2
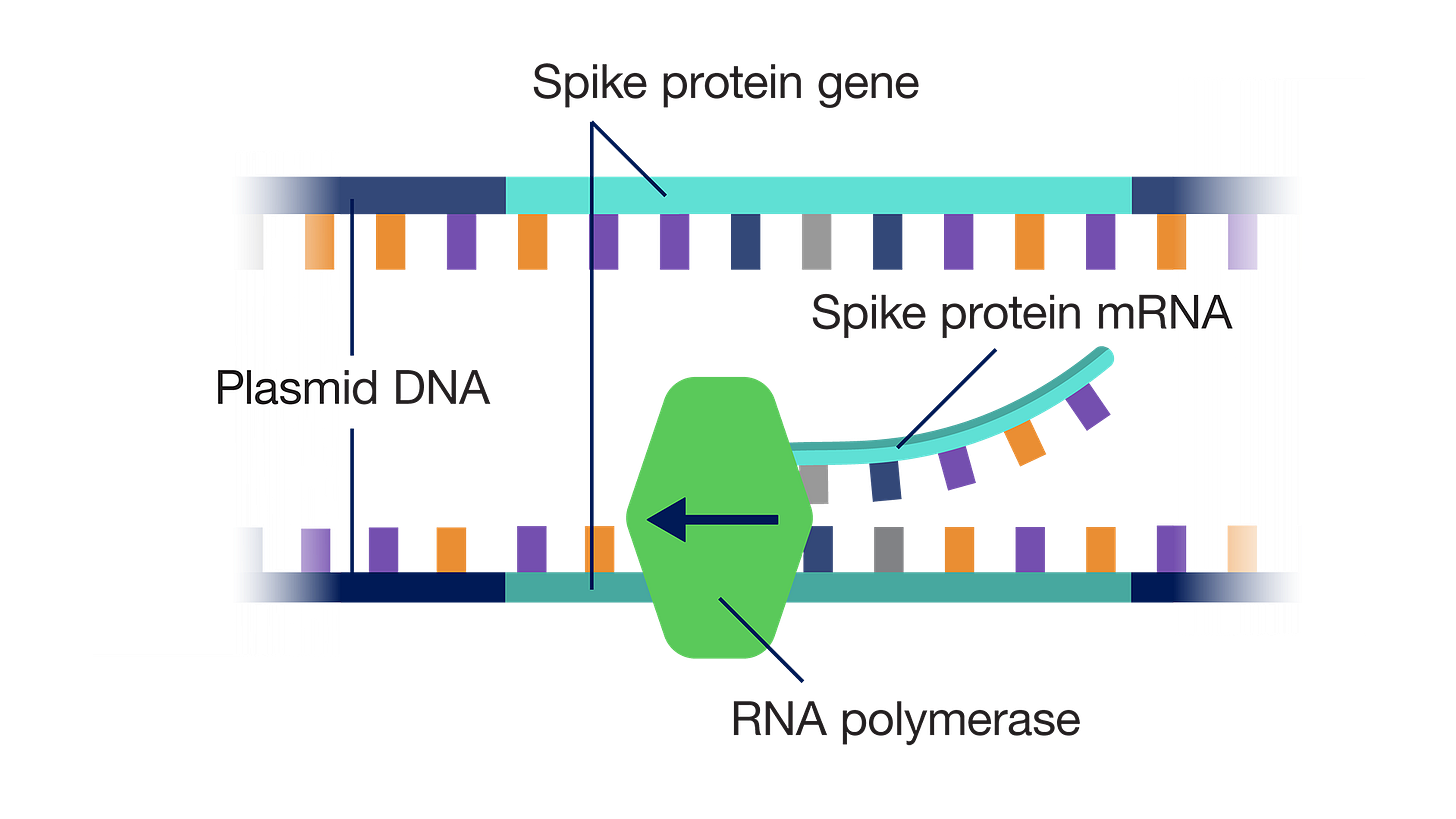
The introduction of 'Process 2', a commercial product, brings worries regarding DNA plasmid fragment contamination. This contamination is attributed to the use of e-coli during manufacture. These bacteria are naturally found in the gut bacteria.
The thorny issue of ‘PROCESS 2’ (the commercial scaling of the product manufactured by Pfizer) which used a completely different process than that used for the product in the clinical trial (Process 1 - PCR manufacture) on which the Emergency Use Authorization was granted, was not compliant with Good Manufacturing Practice (GMP). Note the FOIA’d national contracts with Pfizer
Process 2 is difficult to illustrate, but here are some illustrations from the National Human Genome Research Institute with my captioning -


SPIKE PROTEIN DISEASE
We then delved into the concept of Spike Protein Disease, which has become a cause for concern. This condition arises from the spike proteins produced by the Pfizer vaccine and their potential impact on the human body.
What can we do to rid our bodies of the Spike Protein?
Lastly, we introduced The Wellness Company and the FLCCC (Front Line COVID-19 Critical Care) protocols. These protocols have gained recognition for their effectiveness in managing ‘long’ COVID-19 cases and promoting overall wellness.